The weekly report on research and polls
by Greg J. Neimeyer, Ph.D
 Researchers have found that idle periods of cognitive “down” time- when our minds are “blank” and we are thinking of nothing in particular may, in fact, be particularly telling.┬á The network of brain regions that are active when we are otherwise “inactive” may turn out to hold the key to the differences between normal and abnormal brain function, as recent research has demonstrated in a comparative study of individuals suffering from Parkinson’s and Alzheimer’s disease.
Researchers have found that idle periods of cognitive “down” time- when our minds are “blank” and we are thinking of nothing in particular may, in fact, be particularly telling.┬á The network of brain regions that are active when we are otherwise “inactive” may turn out to hold the key to the differences between normal and abnormal brain function, as recent research has demonstrated in a comparative study of individuals suffering from Parkinson’s and Alzheimer’s disease.
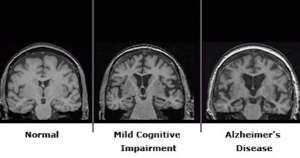
This research measured the level of glucose consumption in different regions of the brain. Measurements of how much glucose brain cells consume reveal that one key brain network that is active during otherwise idle periods of cognitive activity, called the “default mode network”, undergoes rapid losses in levels of activity in people with Alzheimer’s disease, according to a report in the February 9, Proceedings of the National Academy of Sciences. In contrast, the network remains largely intact during the early stages of Parkinson’s disease.
Scientists continue to debate the network’s role, but the latest evidence adds to the growing data linking a breakdown in this network with a wide variety of brain disorders and diseases.
Researchers originally discovered the default mode network using a brain scan procedure known as functional magnet resonance imaging, or fMRI. fMRI measures blood flow, which is an indirect gauge of brain cell activity. Active areas “light up” in red, suggesting hyper-metabolic neocortical activity in those brain regions. Dr. David Eidelberg, a neuroscientist at the Feinstein Institute for Medical Research in Manhasset, N.Y., and his colleagues decided instead to use a more direct brain imaging technique to measure how much glucose regions of the brain were consuming during different phases of cortical activity. This procedure, known as a PET scan, provides a more direct assessment of brain activity because more active brain cells burn more glucose.
In their study the scientists analyzed PET scans of the brains of Parkinson’s and Alzheimer’s patients. The scans of Parkinson’s patients showed that the default mode network was no longer the primary resting network. “There was a new sheriff in town,” in the form of a new neural network not seen in healthy people, Eidelberg says. In the early stages of Parkinson’s disease, this new abnormal network dominated resting brain activity, but otherwise didn’t interfere with the default mode network. As the disease progressed, however, and the patients began to develop dementia, the integrity of the resting network began to decay and the activity broke down. Importantly, its activity could be partially restored by treating patients with levodopa, a drug that converts to dopamine in the body and has long been used in the treatment of Parkinson’s.
To determine the role of the default network in Alzheimer’s disease, the researchers studied PET scans from a database compiled by the Alzheimer’s Disease Neuroimaging Initiative.┬á Alzheimer’s disease patients also had a new abnormal dominant resting network in their brains, but it was different from the one seen in Parkinson’s disease patients. In addition, unlike in Parkinson’s patients, the default mode network of Alzheimer’s patients had already started to decay even in people with mild symptoms who had not yet been diagnosed as having Alzheimer’s disease. ┬áAnd over the course of the subsequent 2 years, as the disease progressed, so too did the levels of inactivity and breakdown in the default mode networks in the Alzheimer’s patients.
The differences between the Parkinson’s patients and the Alzheimer’s patients may stem from the nature of the two diseases. ┬áIn Parkinson’s disease, people lose brain cells that make dopamine, which helps coordinate communication. Restoring dopamine facilitates communication in the brain networks. But in Alzheimer’s disease, brain cells in critical regions of the default mode network die. That damage probably cannot be reversed and function cannot be restored, as it can in the case of Parkinson’s.
PET scans may one day be helpful in screenings or early diagnosis of various brain diseases. And eventually they may be useful, too, in monitoring the effectiveness of medications or other forms of treatment designed to slow or repair the otherwise insidious deterioration associated with these neurocognitive disorders.
